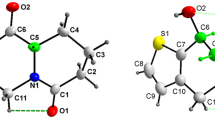
Abstract
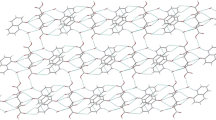
The X-ray structure analyses of 4-hydroxy-1-methylquinolin-2(1H)-one (1), 6-ethyl-4-hydroxy-2H-pyrano[3,2-c]quinoline-2,5(6 H)-dione (2), (E)-4-(2-benzylidene-hydrazineyl)quinolin-2(1H)-one (3), and diethyl (E)-2-(2-(1-methyl-2-oxo-1,2-dihydroquinolin-4-yl)hydrazineylidene)-succinate (4), were carried out. The aforementioned compounds showed strong intramolecular hydrogen bonds which play important roles in the crystal packing of them.
Graphical Abstract










Similar content being viewed by others
Data Availability
Data sharing not applicable to this article as no data-sets were generated or analysed during the current study.
Notes
Distance for pi–pi interaction defined by the distance between the L.S. plane of the molecule and the center of gravity of the symmetry related molecule, generated by the given symmetry operator.
Distance for pi–pi interaction defined by the distance between the L.S. plane of the molecule and the center of gravity of the symmetry related molecule, generated by the given symmetry operator.
Distance for π–π interaction defined by the distance between the L.S. plane of the molecule and the center of gravity of the symmetry related molecule, generated by the given symmetry operator.
References
Akhtar MJ, Yar MS, Khan AA, Ali Z, Haider MR (2017) Recent advances in the synthesis and anticancer activity of some molecules other than nitrogen containing heterocyclic moeities. Mini-Rev Med Chem 17(17):1602–1632
Arafa RK, Hegazy GH, Piazza GA, Abadi AH (2013) Synthesis and in vitro antiproliferative effect of novel quinoline-based potential anticancer agents. Eur J Med Chem 63:826–832
Lee H-Y, Chang C-Y, Su C-J, Huang H-L, Mehndiratta S, Chao Y-H, Hsu C-M, Kumar S, Sung T-Y, Huang Y-Z (2016) 2-(Phenylsulfonyl) quinoline N-hydroxyacrylamides as potent anticancer agents inhibiting histone deacetylase. Eur J Med Chem 122:92–101
Afzal O, Kumar S, Haider MR, Ali MR, Kumar R, Jaggi M, Bawa S (2015) A review on anticancer potential of bioactive heterocycle quinoline. Eur J Med Chem 97:871–910
Ramesh M, Mohan P, Shanmugam P (1984) A convenient synthesis of flindersine, atanine and their analogues. Tetrahedron 40(20):4041–4049
Chen I-S, Tsai I-W, Teng C-M, Chen J-J, Chang Y-L, Ko F-N, Lu MC, Pezzuto JM (1997) Pyranoquinoline alkaloids from Zanthoxylum simulans. Phytochemistry 46(3):525–529
Wabo HK, Tane P, Connolly JD, Okunji CC, Schuster BM, Iwu MM (2005) Tabouensinium chloride, a novel quaternary pyranoquinoline alkaloid from Araliopsis tabouensis. Nat Prod Res 19(6):591–595
Magesh CJ, Makesh SV, Perumal PT (2004) Highly diastereoselective inverse electron demand (IED) Diels–Alder reaction mediated by chiral salen–AlCl complex: the first, target-oriented synthesis of pyranoquinolines as potential antibacterial agents. Biorg Med chem lett 14(9):2035–2040
Siliveri S (2017) Design, synthesis and antibacterial evaluation of pyrano[3,2-H]quinoline carbonitriles. Int J Gre Pharm (IJGP) 11(03):423–429
Martínez-Grau A, Marco J (1997) Friedländer reaction on 2-amino-3-cyano-4H-pyrans: synthesis of derivatives of 4H-pyran[2,3-b]quinoline, new tacrine analogues. Biorg Med chem lett 7(24):3165–3170
Kamperdick C, Van NH, Van Sung T, Adam G (1999) Bisquinolinone alkaloids from Melicope ptelefolia. Phytochemistry 50(1):177–181
Chen J-J, Chen P-H, Liao C-H, Huang S-Y, Chen I-S (2007) New phenylpropenoids, bis (1-phenylethyl) phenols, bisquinolinone alkaloid, and anti-inflammatory constituents from Zanthoxylum integrifoliolum. J Nat Prod 70(9):1444–1448
Isaka M, Tanticharoen M, Kongsaeree P, Thebtaranonth Y (2001) Structures of cordypyridones A–D, antimalarial N-hydroxy-and N-methoxy-2-pyridones from the insect pathogenic fungus Cordyceps nipponica. J Org Chem 66(14):4803–4808
Koizumi F, Fukumitsu N, Zhao J, Chanklan R, Miyakawa T, Kawahara S, Iwamoto S, Suzuki M, Kakita S, Rahayu ES (2007) YCM1008A, a novel Ca^ 2^+-signaling inhibitor, produced by Fusarium sp. YCM1008. J Antibiot 60(7):455
El-Agrody AM, Abd-Rabboh HS, Al-Ghamdi AM (2013) Synthesis, antitumor activity, and structure–activity relationship of some 4H-pyrano[3,2-h]quinoline and 7H-pyrimido[4′,5′:6, 5]pyrano[3,2-h] quinoline derivatives. Med Chem Res 22(3):1339–1355
Hammouda MA, El-Hag FAA, El-Serwy WS, El-Manawaty M (2015) Synthesis and characterization of new fused 4H-pyranquinoline carbonitrile derivatives with anticipated antitumor biological activity. Res J Pharm Bio Chem Sci 6(1):200–208
Fouda AM (2017) Halogenated 2-amino-4H-pyrano[3,2-h]quinoline-3-carbonitriles as antitumor agents and structure—activity relationships of the 4-,6-,and 9-positions. Med Chem Res 26(2):302–313
Maalej E, Chabchoub F, Oset-Gasque MJ, Esquivias-Pérez M, González MP, Monjas L, Pérez C, de los Ríos C, Rodríguez-Franco MI, Iriepa I (2012) Synthesis, biological assessment, and molecular modeling of racemic 7-aryl-9,10,11,12-tetrahydro-7H-benzo[7,8]chromeno [2, 3-b]quinolin-8-amines as potential drugs for the treatment of Alzheimer’s disease. Eur J Med Chem 54:750–763
Nesterova I, Alekseeva L, Andreeva N, Golovina S, Granik V (1995) Synthesis and study the pharmacological activity of derivatives of 5-dimethylaminopyrano[3,2-c]quinolin-2-ones. Pharm Chem J 29(2):111–114
Knorr L (1986) Synthetische Versuche mit dem Acetessigester. J Liebig Ann Chem 236:69–115
Staskun B (1964) The conversion of benzoylacetanilides into 2- and 4-hydroxyquinolines. J Org Chem 29:1153–1157
Stadlbauer W, Hojas G (2004) Ring closure reactions of 3-arylhydrazonoalkyl-quinolin-2-ones to 1-aryl-pyrazolo[4,3-c]quinolin-2-ones. J Heterocycl Chem 41:681–690
Elbastawesy MAI, Ramadan M, El-Shaier YAMM, Aly AA, Abuo-Rahma G, El Din A (2020) Arylidenes of Quinolin-2-one scaffold as Erlotinib analogues with activities against leukemia through inhibition of EGFR TK/ STAT-3 pathways. Biorg Chem 96:103628
Elbastawesy MAI, Aly AA, Ramadan M, Elshaier YAMM, Youssif BGM, Brown AA, Abuo-Rahma G, El-Din A (2019) Novel pyrazoloquinolin-2-ones: design, synthesis, docking studies, and biological evaluation as antiproliferative EGFR-TK inhibitors. Biorg Chem 90:103045
Sheldrick GM (2015) Integrated space-group and crystal-structure determination. Acta Crystallogr A71:3–8. https://doi.org/10.1107/S2053273314026370
Sheldrick GM (2015) Crystal structure refinement with SHELXL. Acta Crystallogr C71:3–8. https://doi.org/10.1107/S2053229614024218
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Aly, A.A., Nieger, M., Bräse, S. et al. X-ray Structure Analyses of 4-Hydroxy-1-Methylquinolin-2(1H)-One, 6-Ethyl-4-Hydroxy-2 H-Pyrano[3,2-c]Quinoline-2,5(6H)-Dione, (E)-4-(2-Benzylidene-Hydrazineyl)Quinolin-2(1H)-One and Diethyl (E)-2-(2-(1-Methyl-2-Oxo-1,2-Dihydro-Quinolin-4-yl)Hydrazineylidene)Succinate. J Chem Crystallogr 53, 38–49 (2023). https://doi.org/10.1007/s10870-022-00939-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-022-00939-z